
RDIF
- The Russian coronavirus vaccine probably won’t be available in the US or UK any time soon.
- The shot was 91.6% effective at preventing COVID-19, according to data published Tuesday.
- Going through the US regulatory process is “not frankly a priority for us,” Kirill Dmitriev, CEO of the Russian Direct Investment Fund told Insider.
- Visit Business Insider’s homepage for more stories.
Russia’s coronavirus vaccine is highly effective, but it won’t be available in the US or UK any time soon, a top Russian official told Insider.
Applying for regulatory approval in the US and UK isn’t a priority, a leader of Russia’s vaccine program said in an exclusive interview on Tuesday. Russia has instead struck deals to sell doses to countries that are more receptive of the vaccine, he said.
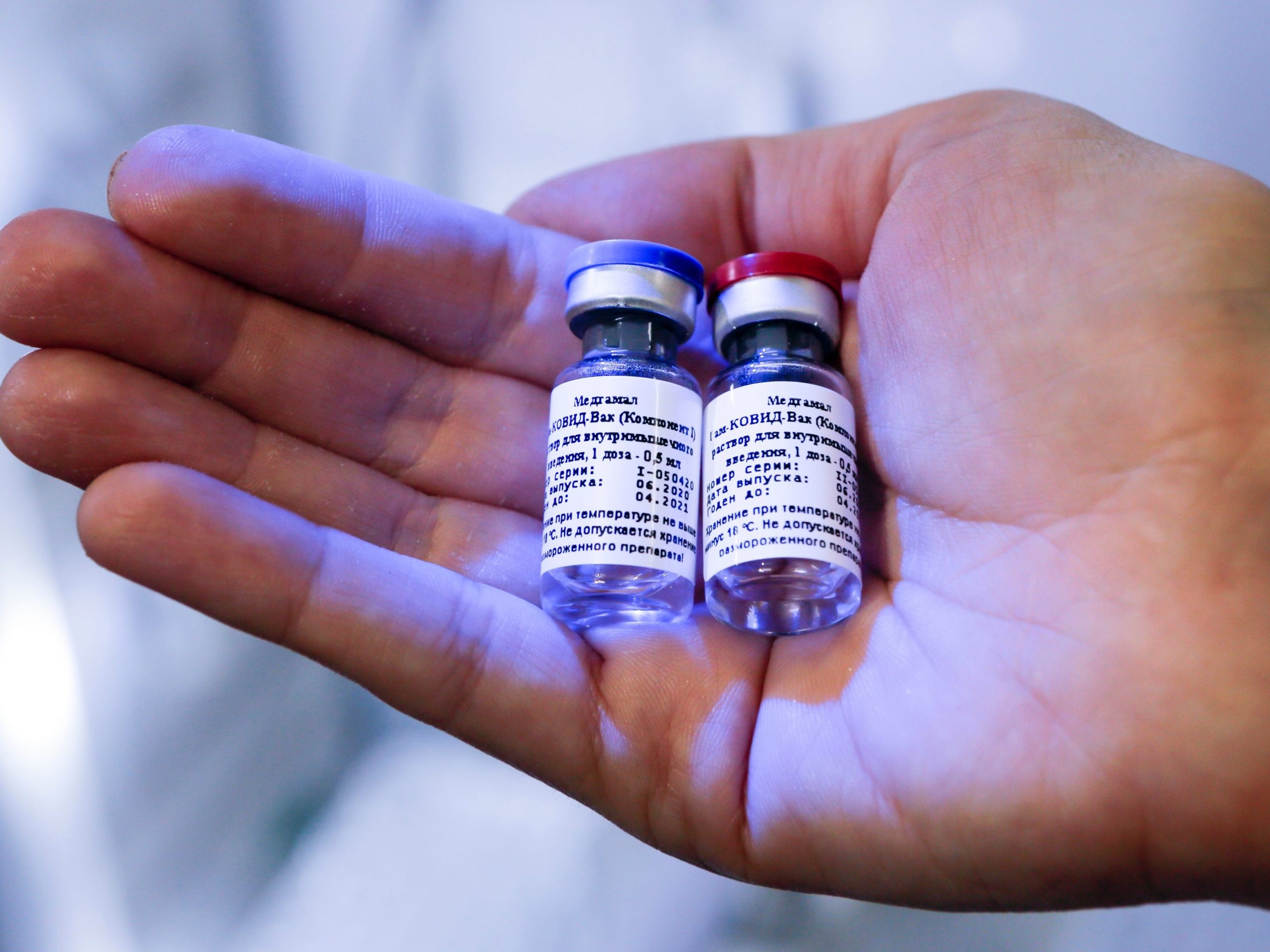
The two-dose vaccine, dubbed Sputnik V, was 91.6% effective at preventing COVID-19 in a trial of nearly 20,000 volunteers, according to interim results published Tuesday in The Lancet, a top medical journal. While the vaccine has already been authorized in more than a dozen countries, don’t expect decisions anytime soon from US or British health regulators.
Going through the US regulatory process is “not frankly a priority for us,” said Kirill Dmitriev, CEO of the Russian Direct Investment Fund. He added that there have not been discussions with regulators at the US Food and Drug Administration, the agency in charge of reviewing medicines.
The RDIF is one of the world’s largest sovereign wealth funds, and has overseen and financed the development of Sputnik V. While scientists at the Gamaleya Institute in Russia developed the shot, the RDIF has taken leadership on the final stages of development, including working with regulators, negotiating supply deals, and ramping up manufacturing.
While the US and UK markets aren't priorities, the European Union remains a possibility, Dmitriev said. Germany's chancellor Angela Merkel has offered help in applying to the European Medicines Agency, Dmitriev said.
Read more: What's coming next for COVID-19 vaccines? Here's the latest on 11 leading programs.
'Requires two to tango,' Dmitriev says
Leaders of the Russian vaccine program spoke in August 2020 with officials of Operation Warp Speed, the ambitious vaccine initiative launched by President Donald Trump, Dmitriev said. The Russian team offered to work together, particularly in testing Sputnik V in combination with other vaccine frontrunners.
"It didn't really go much, I think, because of obvious political constraints of working with Russia," he said.
"We are open to this, but it requires two to tango," Dmitriev added. "We are putting our openness out there through Business Insider and see if the US is willing to take on this."
The Russian program gained international attention on August 11, 2020, when President Vladimir Putin announced the shot was approved, even though late-stage studies had yet to be completed. While vaccine experts have expressed skepticism about the program since, Dmitriev hopes the data published in The Lancet will earn more trust in the shot.
In the UK, Dmitriev said applying for approval will depend on succeeding with a planned trial that uses Sputnik V in combination with a vaccine developed by AstraZeneca and the University of Oxford. That combination study could start enrolling volunteers as soon as next week, he said.
"If that shows high level of efficacy, we will be happy to approach the UK with that, obviously jointly with AstraZeneca," he said.
That study will take months to run, and before that point Dmitriev said Russia will not apply for UK authorization.
Russia expects to produce 700 million doses in 2021
Alexander Zemlianichenko Jr/Russian Direct Investment Fund via AP
Russia has already reached a handful of supply deals with other countries, none of them major Western powers. Dmitriev said he expects to produce 700 million doses of the 2-dose shot in 2021 and the program is already "oversubscribed" with supply deals with other nations.
"For now, we are focused on markets that look at who we are on the merit of data, on the merit of our platform," Dmitriev said, "and obviously something with UK, something with Europe, something with US would have major political overtones not from us but from them."
Sixteen foreign countries or sovereign states have already OK'd Russia's shot: Belarus, Argentina, Bolivia, Serbia, Algeria, Palestine, Venezuela, Paraguay, Turkmenistan, Hungary, United Arab Emirates, Iran, Guinea, and Tunisia. Dmitriev said he expects Sputnik V to be registered in 25 nations by the end of next week.
